- Wind speed Air volume number of air changes
- Unidirectional flow/non-unidirectional flow
- Temperature and humidity
- Differential pressure
- Suspended particles
- Plankton
- Sedimentation bacteria
- noise
- intensity of illumination
Firstly, the term GMP originated from the pharmaceutical industry, and was later borrowed by the medical device industry. The medical device production quality management Standard and its appendix stipulate and require medical device manufacturers to establish and improve the quality management system in accordance with relevant regulations.
The contents include organization and personnel, plant and facilities, equipment, document management, design and development, procurement, production management, quality control, sales and after-sales service, nonconforming product control, adverse event monitoring, analysis and improvement, etc.
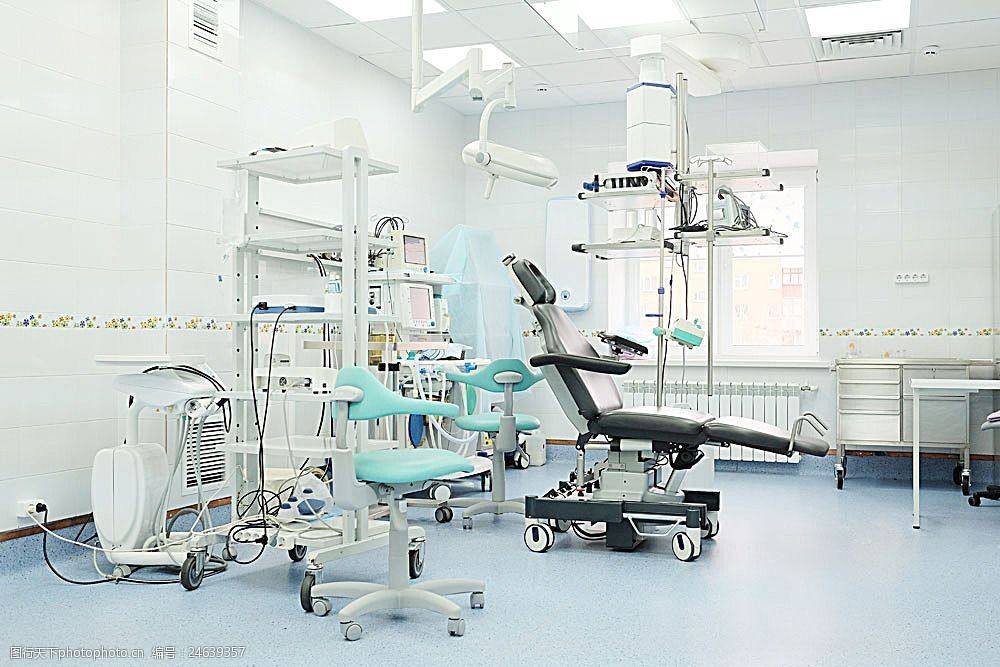
The following summarizes eight points to tell you about the environmental requirements in the medical device workshop

Wind speed Air volume number of air changes
The cleanliness of clean rooms and clean areas is mainly achieved by sending enough clean air to discharge and dilute the particulate pollutants produced in the room. Therefore, it is necessary to determine the air supply volume, average wind speed, air supply uniformity, air flow direction and flow pattern of clean rooms or clean facilities.
Unidirectional flow/non-unidirectional flow
Unidirectional flow mainly relies on clean airflow to push and displace the polluted air in the room and area to maintain the cleanliness of the room and area. Therefore, the wind speed and uniformity of the air supply section are important parameters affecting the cleanliness. Higher and more uniform cross-sectional wind speeds can remove pollutants from indoor processes faster and more effectively, so they are the main detection items of concern.
Non-unidirectional flow mainly relies on the clean air to dilute and dilute indoor and regional pollutants to maintain their cleanliness. Therefore, the greater the number of air changes, the more reasonable the flow pattern, the more significant the dilution effect, and the corresponding improvement in cleanliness.
Temperature and humidity
The measurement of temperature and humidity in clean rooms or clean facilities is usually divided into two grades: general test and comprehensive test. The second level is suitable for static or dynamic comprehensive performance testing. This kind of test is suitable for the temperature, humidity performance requirements are more strict occasions.
This test is carried out after air flow uniformity detection and air-conditioning system adjustment. At the time of the test, the air-conditioning system was fully operational and conditions were stable. At least one humidity sensor is set in each humidity control area, and sufficient stabilization time is given to the sensor.
Differential pressure
The purpose of this test is to verify the ability of the completed facility to maintain a defined pressure differential between the completed facility and its surroundings and between Spaces within the facility. This test applies to all three occupancy states. This test needs to be done regularly. The test of pressure difference should be in the condition that all doors are closed, from high pressure to low pressure, from the plane layout and the most distant outside the inner room, outward test in turn; Different grades of adjacent clean rooms with holes connected should have reasonable air flow direction at the hole and so on.

Suspended particles
Plankton
The minimum number of sampling points corresponds to the number of sampling points of suspended particles. The measuring point in the working area is about 0.8-1.2m above the ground, and the measuring point in the air supply port is about 30cm away from the air supply surface.
The measuring point can be added to the key equipment or key working activity range, and each sampling point is generally sampled once. After all sampling, culture dishes should be placed in a constant temperature incubator for no less than 48 hours. Control experiments should be conducted for each batch of culture medium to test whether the culture medium is contaminated.

Sedimentation bacteria
Settlement bacteria workspace point position from the ground 0.8-1.2 m or so, will have the preparation of good dishes in a sampling point, open a dish cover, make its exposure to the stipulated time, then a dish cover, put in the dish in the constant temperature incubator culture, time is not less than 48 hours, each batch of medium should have a control experiment, see if medium pollution.
noise
If the measurement height is about 1.2 meters from the ground and the clean room area is less than 15 square meters, only 1 point in the center of the room can be measured. The area is more than 15 square meters, but also should be measured diagonal 4 points, 1 meter away from the side wall, measuring points toward each Angle.
intensity of illumination
The measuring point plane is about 0.8 meters from the ground, and the measuring point is arranged according to the spacing of 2 meters. The measuring point of the room within 30 square meters is 0.5 meters from the side wall, and the measuring point of the room over 30 square meters is 1 meter from the wall.